Image: Shutterstock

The first periodic table, designed by a Russian scientist named Dmitri Mendeleev, dates back to 1869. It ranks the elements in an easy-to-read method according to their physical and chemical attributes. This post contains a periodic table for kids that you can use to teach your little one about the various elements and their properties (1).
The elements are arranged according to their atomic number, and scientists have adopted this arrangement worldwide to help classify and rank the elements. Learning about the periodic table can help your kids with chemistry and other scientific concepts they will encounter as they progress in their education. Read on to learn more about the periodic table, the right way to read it, and the reason behind it.
Key Pointers
- The periodic table is designed based on physical and chemical characteristics.
- The elements are arranged in 7 rows and 18 columns in the table.
- It is easy for the children to understand and remember the characteristics and attributes of the elements.
Why Is It Called The Periodic Table?
The elements in the periodic table are arranged in rows and columns
. There are 18 columns known as groups and seven rows called periods. The periodic table gets its name from the seven periods (2).
Each element is placed along with other elements sharing the same characteristics, such as non-metal and noble gas. These elements are considered part of the same group. Elements in a group share similar characteristics, including the way they react to other elements.
The Periodic Table
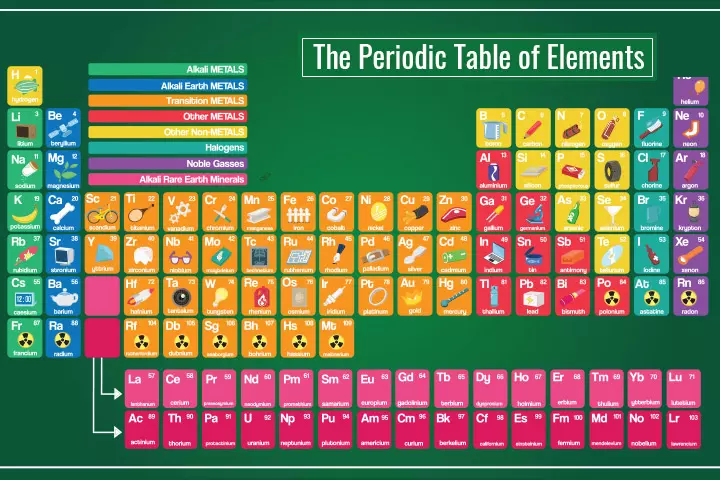
Image: Shutterstock
How To Read The Periodic Table?
There are various ways in which the elements in the periodic table are placed and characterized. An element can be considered a part of a row and column with further sub-classifications across rows, columns, or the table. Therefore, the interpretations are vast and could vary based on one’s purpose. Below we explain the basic forms of classification of elements in the periodic table.
1. Fundamental division
The periodic table has the following fundamental attributes.
- There are seven rows or periods. These elements share physical properties, such as malleability and conductivity. Two rows of elements from the atomic number 57 to 71 (lanthanides) and 89 to 103 (actinides) are usually grouped below the main table.
- There are 18 columns or groups. These groups share similar atomic structures and characteristics, such as valency (combining capacity of the atoms).
2. Sub-groups
The elements in the periodic table can be classified into the following three basic types or sub-groups.
- Metals: These elements share similar characteristics of melting point, malleability (flexibility), and conductivity (transmission of electricity and heat).
- Non-metals: Non-metal elements are typically non-conductors or poor conductors of heat and electricity. They are not malleable and lack the characteristic shine or luster of metals.
- Metalloids: These are also called semi-metals in chemistry. Metalloid elements have characteristics that lie in between metals and non-metals. For instance, metalloids could be shiny like metals but could be poor conductors of heat like non-metals. Some metalloids could be malleable like metals, while others could have poor malleability like non-metals.
 Quick fact
Quick fact3. Column groups
The elements can also be read as part of a group (column). Elements in each column usually share similar characteristics. Below are the names of various columns.
- Column 1: Hydrogen and alkali metals
- Column 2: Alkaline earth metals
- Columns 3 to 12: Transition metals
- Columns 13 to 16: Post-transition metals, metalloids, and non-metals
- Column 17: HalogensiNon-metallic chemical elements that react with metals to form a salt.
- Column 18: Noble gases
The columns 3 to 16 also consist of lanthanides, also known as rare-earth elements, and actinides. The properties of these elements differ from the rest. Thus, they are placed at the bottom of the periodic table.
The arrangement of molecules in an element can be analyzed using its electron configuration, which can also be used to determine the number of valence electrons present in the atom. The valence electrons are the outermost electrons in an atom that are involved in chemical bonding and are crucial in understanding the behavior of chemical equations and formulas. By referencing the periodic table and identifying the column group number of the element, one can determine the number of valence electrons present. This information is essential for predicting the reactivity and bonding properties of the element.
 Did you know?
Did you know?4. Atomic number, atomic mass, and atomic weight
The elements of the periodic table can also be interpreted based on their atomic number, atomic mass, and atomic weight. Below are their definitions (3) (4).
- Atomic number: It is the number of protons (positively charged particles) present in the nucleusiThe positively charged, central part of an atom, consisting of protons and neutrons. of an element’s atom. It is often represented by the letter Z.
- Atomic mass: It is the number of protons and neutrons (neutrally charged particles) present in the nucleus of an element’s atom. It is often represented by the letter A.
- Atomic weight: An element could have multiple isotopes, which are different forms of the element with the same atomic number (same number of protons) but different number of neutrons. The atomic weight is the average of the atomic masses of all the isotopes of the element.
The following interpretations and characteristics of elements can be observed based on their atomic number, atomic mass, and atomic weight.
- Each element will typically contain one more proton than the one before it. For example, Helium (He) has an atomic number of 2, which is 1 more than the previous element Hydrogen (H). Therefore, the atomic number (the number of protons) will increase as you go down the table.
- Since the number of protons increases, atomic mass and weight increase as you go down a period or across a group, with some notable exceptions.
- Groups of elements typically have the same color scheme and will share many physical properties.
- Some element groups could display changes in physical properties as their atomic masses change. For instance, noble gases tend to become denser as their atomic mass increases.
- The atomic number (number of protons) of an element will always remain the same. However, there could be several undiscovered isotopes of the element. Therefore, the atomic weight could be subject to change, and the one mentioned on the periodic table is generally considered provisional.
Facts About The Periodic Table For Kids
The periodic table is a fascinating tool to categorize elements and has interesting facts about it, too. Here are some fun facts about the periodic table for children (5) (6).
- The periodic table is a dynamic scientific tool designed to evolve and accommodate elements that get discovered. New elements can be categorized and positioned in the existing periodic table. In fact, many elements present in the modern periodic table were not part of the original periodic table in 1869.
- The periodic table has many elements and their elemental symbols contain almost every letter in the alphabet except the letter “J.” New elements are still being discovered, and it is quite likely “J” could be assigned as an elemental symbol.
- Periodicity is the term used to describe the recurring trends or patterns in the chemical and physical properties of elements in the periodic table. The arrangement of elements in the periodic table enables scientists to identify patterns or trends in the properties of elements. Some of the common periodic trends include atomic radius, electronegativity, ionization energy, electron affinity, and oxidation states.
- As of 2025, there are 118 elements in the modern periodic table. 94 elements are naturally occurring elements found on Earth. 24 elements are human-made and are known as synthetic elements.
 Did you know?
Did you know?- The most recently added elements to the periodic table were Nihonium, Moscovium, Tennessine, and Oganesson. These elements were added in 2016 after a five-month review by the International Union of Pure and Applied Chemistry (IUPAC).
Frequently Asked Questions
1. What is the importance of the periodic table?
Periodic tables help children know about various elements, their symbols, physical and chemical properties, and reactivity with one another. Their knowledge about metals isn’t only used in chemistry but also subjects, such as physics, as elements in the periodic table are a part of our daily lives. Knowing these basics makes understanding chemical reactions easier and more exciting.
2. At what age do kids learn about the periodic table?
There’s no fixed age when children can learn the periodic table. Some children can start knowing the basics of the periodic table from as young as five years of age. However, this isn’t a general case.
3. What are some common uses of different elements found on the periodic table?
There are various elements on the periodic table with different uses and characteristics. The most common elements are oxygen, used for respiration; carbon, used in fuels and plastics and found in all living things; hydrogen, an important component of water and also a potential fuel source; and sodium, used for making salt and glass.
4. How can I help my child memorize the periodic table?
You can break the periodic table into smaller sections so it is easier for your child to learn. They can learn the names of the elements with similar properties to remember. You can also use mnemonics or acronyms to make learning easier. Moreover, using flashcards and various media to repeat the information can make it easier for them to memorize the periodic table. You can use technology like YouTube videos or mobile games in an interactive way.
Some interactive techniques can make it easier for the kids to learn the periodic table. Stephanie, a teacher and a blogger, shares her interesting method of making her students learn the periodic table, ”To get them to dive into the actual table and learn a few of the elements, I had them do some periodic table spelling. Basically, they searched around the table for element symbols that spelled out various words. For example, Iron (Fe) and Argon (Ar) spell FeAr. Fear. Some kids were super creative, spelling their own names or really long words. Others attempted sentences (i)!”
5. What fun activities can I use to teach my kids about the periodic table?
Engaging, hands-on activities such as creating element trading cards, using interactive apps and games to learn about the elements, and simple experiments such as creating a baking soda and vinegar reaction can help illustrate the properties of elements and compounds and make learning more enjoyable and memorable.
6. Are there any common trends or patterns found on the periodic table that kids should be aware of?
One of the most important science facts for kids regarding the periodic table is that it holds several trends and patterns. First, the horizontal rows (periods) have an increasing number of electrons while the vertical columns (groups) have elements with similar properties. Next, the properties of each group range from highly reactive metals (group 1) to highly reactive nonmetals (group 17). Noble gases or nonreactive gases form group 18. Children can also learn that the radius of the atom increases from left to right in each period. Moreover, certain elements in periods 6 and 7 onwards are radioactive.
7. What are the first twenty elements in the periodic table for kids?
The first twenty elements in the periodic table are:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulphur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
8. Which is the strongest element in the periodic table?
In terms of individual elements, the strength of an element can be measured by various properties, such as hardness, tensile strength, or other material-specific characteristics. However, Tungsten, also known as heavy stone in Swedish, is the strongest element in the periodic table. It was invented in 1781 and is used in making bullets and missiles, metal evaporation processes, paint production, the fabrication of electron and television tubes, and glass-to-metal seals.
Learning about different metals and their properties is vital for understanding their chemical nature. These simple yet effective ways to understand how metals are classified in a periodic table can help children memorize their order and properties with ease. It can also help children learn how metals react with each other and other elements, such as water, acid, and oxygen. To intrigue young minds more, share these interesting periodic table facts for kids and add to their current knowledge about the world of metals.
Learning about the periodic table can be an engaging way for children to explore science. It can give them information about every element that builds the universe. But children may find it boring when they do not understand the essence of it. So scroll through the infographic below for some fascinating periodic table facts you can share with children to pique their curiosity.
Some thing wrong with infographic shortcode. please verify shortcode syntaxIllustration: How To Explain The Periodic Table To Kids And Facts About It

Image: Stable Diffusion/MomJunction Design Team
Does the Periodic table seem difficult to understand? If yes, then don’t worry, and learn how to perfect the Periodic Table with this helpful video. Get tips and tricks to help you understand and remember the elements.
Personal Experience: Source
MomJunction articles include first-hand experiences to provide you with better insights through real-life narratives. Here are the sources of personal accounts referenced in this article.
i. Great lesson 1: The periodic table [Post 9]https://www.teachinginroom6.com/2014/11/the-periodic-table.html
References
1. Periodic Table of Elements; U.S. National Library of Medicine
2. Periodic Table of Chemical Elements; American Chemical Society
3. Atomic Number and Atomic Mass; Radiation Emergency Medical Management
4. Periodic Table of Elements; Los Alamos National Laboratory
5. IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson; IUPAC
6. Jennifer Chu, An element that’s rare on Earth is found far, far away; Massachusetts Institute of Technology
7. Periodic trends made easy!; Chemistrytalk.org
8. Thorium; Rsc.org
Community Experiences
Join the conversation and become a part of our nurturing community! Share your stories, experiences, and insights to connect with fellow parents.
Read full bio of Beth Sullivan
Read full bio of Soma Sengupta
Read full bio of Harshita Makvana
Read full bio of Praggya Joshi